Your Definitive Guide to Retinal Imaging Biomarkers & Endpoints
The 3rd Annual Retinal Imaging Biomarkers & Endpoints Summit is the premier industry forum dedicated to advancing retinal imaging technologies for biomarker discovery and clinical trial endpoints.
Uniting key experts from biotech, pharma, and academia, the event focuses on optimizing imaging modalities, improving regulatory pathways, and accelerating drug development in ophthalmology. Through interactive discussions, case studies, and the latest data, attendees will explore emerging imaging techniques, AI-driven analysis, and validation strategies.
Join us to collaborate on shaping the future of retinal imaging and advancing precision medicine for retinal diseases.
Join Our Expert Speakers for 3 Days of
Learn from the industry’s leading retinal imaging specialists, data scientists, and AI experts, with speakers from Novartis, REGENXBIO, Novartis, Ocunexus, Bausch + Lomb, Galimedix, and more!
The latest research on multimodal imaging and precision biomarkers to refine endpoints and enhance regulatory success
Harness the power of AI and advanced analytics to refine and optimize patient selection, stratification, and clinical trial success for AMD, GA, DR, IRDs, and glaucoma
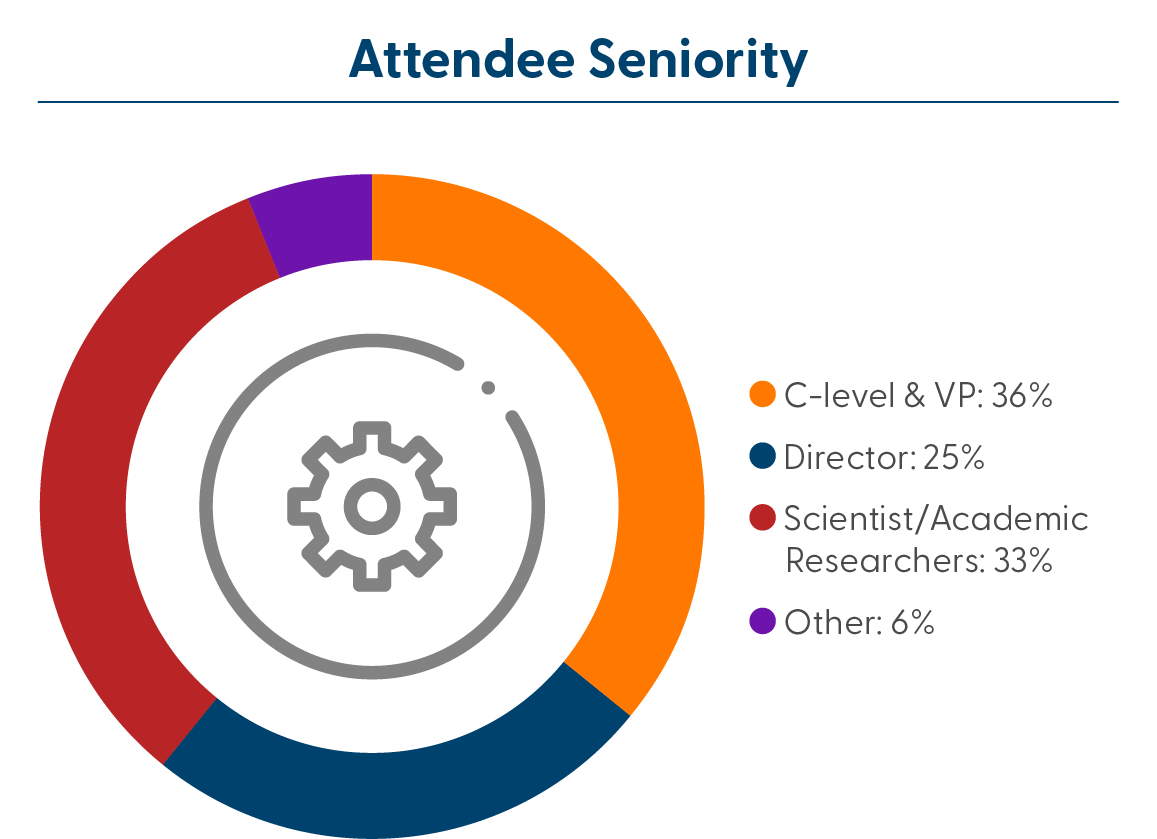
Who Will You Meet?
The 3rd Annual Retinal Imaging Biomarkers & Endpoints Summit connects clinical scientists, imaging scientists, medical directors, R&D, imaging technology and algorithm engineers focusing on spearheading their development.